
Fine Chemicals & Fluorination Technology
DAIKIN Industries produces and offers wide ranged intermediates through an integrated production system that starts with hydrofluoric acid. In addition to offering products on the market, DAIKIN Industries accommodates custom synthesis in response to emerging needs.
Applications of fluorinated compounds, especially fluorinated intermediates are rapidly expanding and used for final products in many Life Science and Industrial applications.
Beyond that DAIKIN Industries provides a series of fluorinating agents called "MEC Reagents" as well as custom synthesis of various fluorochemicals using F2, IF5/Et3N-3HF, and SF4 that can efficiently fluorinate hydroxyl-, carbonyl- and carboxyl- groups, and is particularly suited to selective Difluorination (C=O into CF2).
MEC reagents are safe fluorinating agents for synthesis of new fluoro-compounds and are particularly useful for laboratory and bulk synthesis.
Custom Synthesis
Novel promising fluorochemicals developed in your laboratory from the reagents listed below can be efficiently developed and synthesized in bulk by DAIKIN Industries; utilizing its industrial fluorination technologies.
Intermediates
DAIKIN produces and offers a wide range of intermediates. The selection below provides a first impression of our product range and capabilities.
If you are looking for a fluorinated compound not mentioned in the list, please contact us.
- IntermediatesDetails
-
Chemical name Chemical formula CAS NO TSCA 1H,1H-pentafluoropropanol (5FP) CF3CF2CH2OH 422-05-9 Y 2-(perfluorobutyl)ethanol (C4 Alcohol) F(CF2)4CH2CH2OH 2043-47-2 Y 2-(perfluorohexyl)ethanol (C6 Alcohol) F(CF2)6CH2CH2OH 647-42-7 Y 1H,1H,3H-tetrafluoropropanol CHF2CF2CH2OH 76-37-9 Y 1H,1H,5H-octafluoropentanol H(CF2)4CH2OH 355-80-6 Y 1H,1H,7H-dodecafluoroheptanol H(CF2)6CH2OH 335-99-9 Y 2H-hexafluoro-2-propanol (HFIP) (CF3)2CHOH 920-66-1 Y (perfluorohexyl)ethylene (C6 Olefin) F(CF2)6CH=CH2 25291-17-2 Y perfluorohexyl iodide (C6 Telomer) F(CF2)6I 355-43-1 Y 2-(perfluorohexyl)ethyl iodide F(CF2)6CH2CH2I 2043-57-4 Y 2-(perfluorohexyl)ethyl methacrylate (C6 SFMA Monomer) F(CF2)6CH2CH2OCOC(CH3)=CH2 2144-53-8 Y 2-(perfluorohexyl)ethyl acrylate (C6 SFA Monomer) F(CF2)6CH2CH2OCOCH=CH2 17527-29-6 Y hexafluoroisopropyl methyl ether (CF3)2CHOCH3 13171-18-1 N 2,2-bis(3,4-anhydrodicarboxyphenyl)hexafluoropropane (6FDA) C19H6F6O6 1107-00-2 Y
Fluorination Technology
Beyond fluorinated intermediates, DAIKIN also provides a series of fluorinating agents as well as custom synthesis of various fluorochemicals.
DAIKIN is unique in offering iodine pentafluoride, which has rarely been used in fluorination reactions due to the difficulty of controlling reactivity. We have commercialized this technology and are able to offer this low cost, highly selective process to manufacture a variety of organic compounds.
Fluorinating reagents
Electrophilic fluorinating reagent
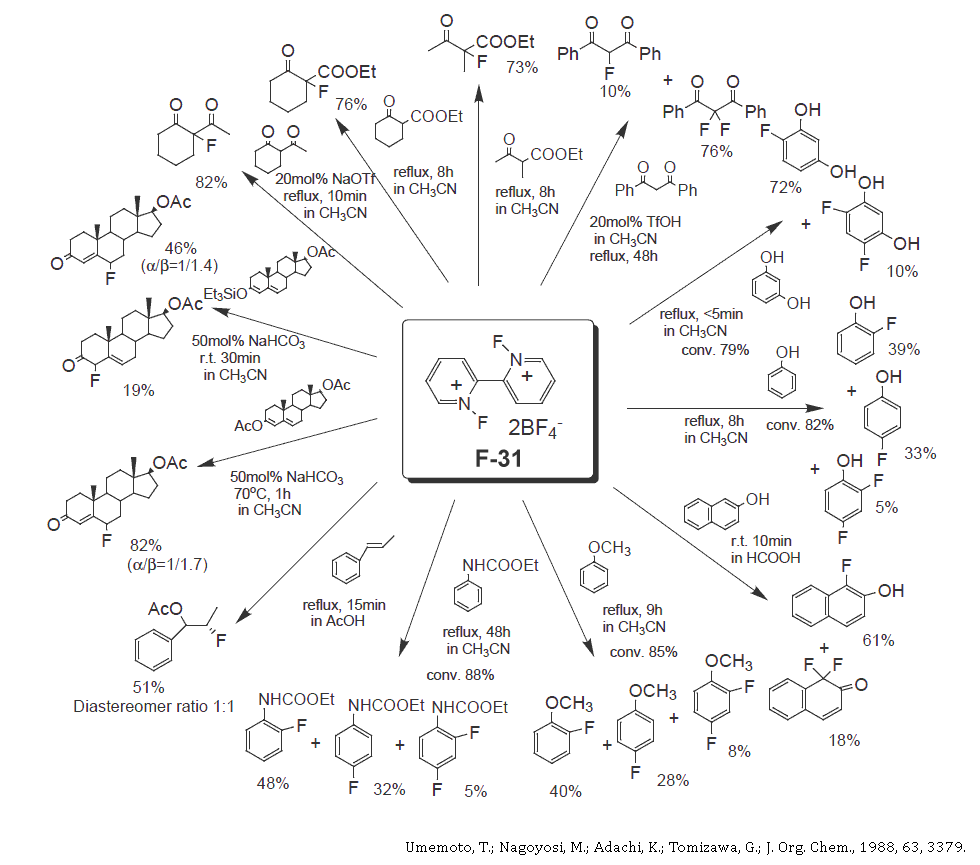
- F-31Details
Electrophilic fluorinating reagent with the most effective fluorine content in its class (available in bulk)
-
Structure and properties
Chemical Name N,N'-Difluoro-2,2'-bipyridinium bis(tetrafluoroborate) Mol Formula C10H8F10N2B2 Mol Weight 367.79 Appearance White Crystal Melting Point 166-168°C (with decomp.) Solubility Acetonitrile 4.7 mg/ml (25°C) Effective F-Content 103g/kg (two F+/Mol Weight) Characteristics
- High Effective Fluorine-Content: 103g/kg (Selectfluor: 53.6g/kg)
- Easy to handle; a glass reactor can be used.
- Cost effective fluorinating agent.
- Easy to remove another product, 2,2'-Bipyridinium bis(tetrafluoroborate) form the fluorination products, as it dissolves in diluted hydrochloric acid.
- Wide variety of application in pharmaceutical and agricultural industries, etc.
- Perfect Recycle System.
Nucleophilic Fluorinating reagent
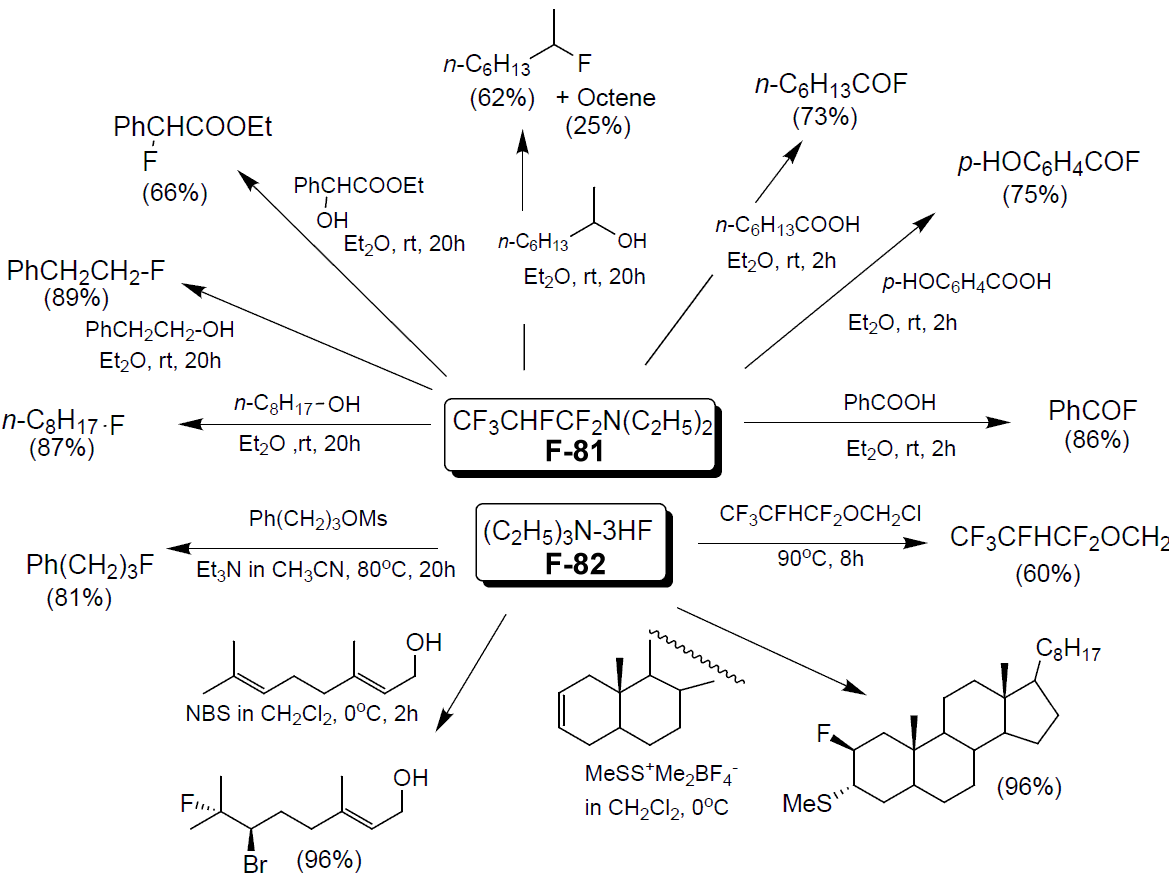
- F-81 / F-82Details
Nucleophilic Fluorinating reagent
-
Structure and properties
Chemical Name
1,1,2,3,3,3-Hexafluoro-1-diethylamino-propane
Chemical Formula
C7H11F6N
Molecular Weight
223.16
Appearance
colorless transparent liquid
Boiling Point
56°C / 58mmHg
Specific Gravity
1.23
CAS No.
309-88-6
Chemical Name
Triethylamine trishydrofluorides
Chemical Formula
C6H18F3N
Molecular Weight
161.21
Appearance
colorless liquid
Boiling Point
70°C / 15mmHg
Specific Gravity
0.989
CAS No.
73602-61-6
Characteristics
- F-81 is a nucleophilic fluorinating reagent with similar reactivity as diethylaminosulfur trifluoride (DAST). F-81 can substitute the hydroxyl group with fluorine under mild conditions.
- F-81 is a source of HF that can be handled much more easily than anhydrous HF, and is a liquid with a high boiling point.
- F-81 and F-82 can both be supplied in bulk.
Direct Fluorination using Elemental Fluorine
This is one of the lowest-cost fluorination processes. DAIKIN Industries has the technology for controlling the extreme reactivity of elemental fluorine. F2 can be an industrial agent for relatively simple compounds that have a clearly identified reactive site.
Fluorination using IF5/Et3N·3HF Complex
The IF5/Et3N·3HF complex is a unique fluorinating reagent which can fluorinate both electrophilic and nucleophilic sites. DAIKIN Industries has commercialized this unique technology and is now able to offer this low cost, highly selective process to manufacture a variety of organic compounds.
- IF5/Et3N·3HFDetails
-
Iodine pentafluoride (IF5) has been rarely used in the fluorination of ordinary organic compounds until recently because of the difficulty in controlling its reactivity. However, Hokkaido University's Professor Yoneda discovered that the use of IF5 in combination with Et3N·3HF salt significantly improves the reaction selectivity and the process as a low-cost and generally applicable industrial fluorination technology, and is using it in the industrial synthesis of a variety of fluorine-containing organic materials.
An extremely unique aspect of the fluorination using IF5/Et3N·3HF salt is that the fluorinating reaction of organic compounds occurs at both nucleophilic and electrophilic sites as illustrated in the scheme below.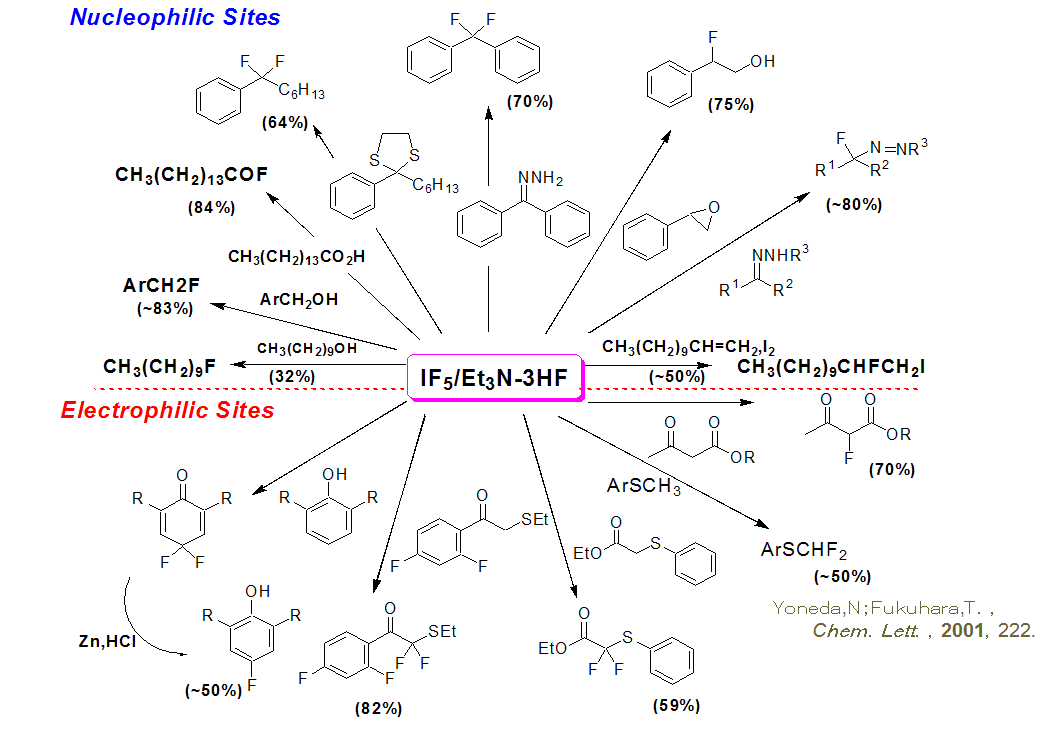
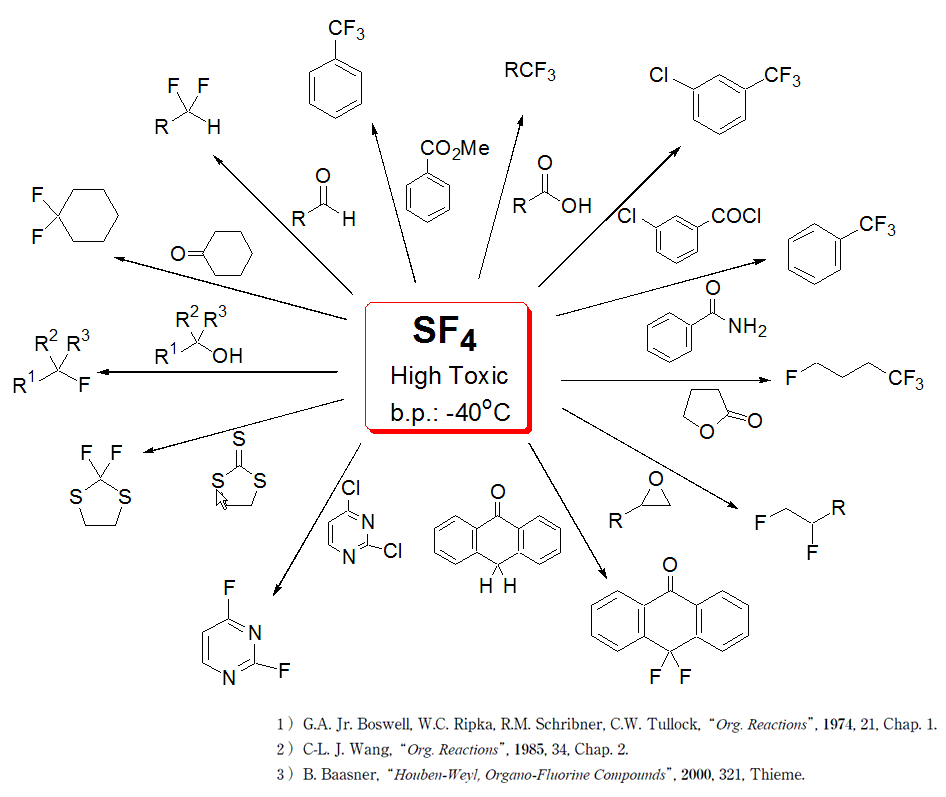
Fluorination using Sulfur tetrafluoride SF4
DAIKIN Industries is committed to manufacturing compounds using Sulfur tetrafluoride on a commercial scale. SF4 is a versatile reagent used to convert a carbonyl into a difluoromethyl group or to obtain a trifluoromethyl from a carboxylic acid.
- SF4Details
-
Sulfur tetrafluoride can be used to efficiently fluorinate hydroxyl-, carbonyl and ester groups. DAIKIN Industries is committed to manufacturing compounds using Sulfur tetrafluoride on commercial scale. SF4 is a versatile reagent used to convert a carbonyl into a difluoromethyl group or to obtain a trifluoromethyl from a carboxylic acid, see scheme below.
Fluorinated Building Blocks
A wide range of building blocks can be derived from DAIKIN Industries' raw materials (tetrafluoroethylene, hexafluoropropene, hexafluoroacetone, tetrafluorooxetane and perfluoroalkyliodides).
